Abstract
INTRODUCTION
Sparse data exist regarding I+V in an off-trial setting, particularly in patients (pts) with progression of disease on either I or V as single agent treatment. The 1-year overall survival (OS) rates with V monotherapy following I range from 75%-91% (Eyre et al, BJH 2019; Jones et al, Lancet Oncol 2018); however, longer-term follow-up of these or other patient cohorts treated with V after I has not yet been reported. Effective options in double-refractory pts are lacking when a clinical trial is not feasible. Here, we report our experience with I+V in these patient groups.
METHODS
After IRB approval, we reviewed the records of pts seen at Mayo Clinic (between 4/2012-1/2021) who received I+V therapy off-trial for CLL progression. Pts treated with I+V for Richter's transformation were excluded. Time to next treatment (TTNT) was measured from I+V treatment start date to the date of next treatment start or last known treatment status with death as a competing risk. OS was measured from I+V start date until date of death or when the patient was last known alive; OS was analyzed using the Kaplan-Meier method and Cox proportional hazards model.
RESULTS
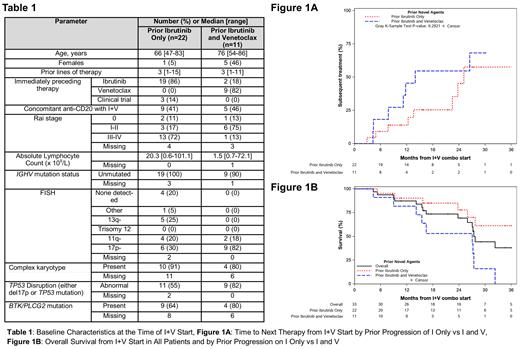
Thirty-three pts with progressive CLL were treated with I+V (median duration 13.3 months [95% CI: 7.1 months-not estimable (NE)]). The median age was 70 years (range 47-86), 6 (18%) were women, and 20 (65%) had del(17p) or TP53 mutation. The median number of prior lines of therapy was 3 (range 1-15). Additional characteristics at the time of I+V start are shown in Table 1. Median follow-up from start of I+V was 23.8 months.
Among 22 pts with prior progression on I only (V-naïve), the median duration of I+V was 14.8 months (95% CI 7.6 months-NE). The median duration on either I or V from the start of combination was 20.3 months (95% CI 8.3 months-NE). The median TTNT was 25.1 months (Figure 1A). Four pts proceeded to cellular therapy (alloSCT [n=3], CAR-T [n=1]) while on I+V. Next therapy (n=9) consisted of Richter transformation treatment (n=2), chemotherapy plus continued I+V (n=1), anti-CD20 plus continued I+V (n=1), duvelisib + I (n=1), TP-0903 + I (n=1), reintroduction of I at disease progression after previously stopping I during I+V therapy (n=1), and reintroduction of I (n=1) or V (n=1) at disease progression after previously stopping I+V. Of 9 pts who did not receive subsequent therapy, 4 pts remain on I+V (median duration 16.4 months), 4 pts continued V after discontinuing I (median duration on V from start of I+V was 21.5 months), and 1 pt discontinued I+V following a second cancer diagnosis and died without receiving additional CLL-directed treatment.
Eleven pts received I+V in the double-refractory setting; 9 pts had prior disease progression on sequential I then V and 2 pts had prior disease progression on sequential V then I. The median duration of I+V was 7.5 months (95% CI 4.3 months-NE), and the median duration of either agent from I+V start was 10.8 months (95% CI 7.1 months-NE). The median TTNT was 14.0 months (Figure 1A). Eight pts required additional treatment, consisting of PI3K inhibitor (n=2), anti-CD20 added to I+V (n=1), CAR-T (n=1), anti-CD20 with (n=1) or without (n=1) bendamustine added to continued I, and Richter transformation treatment (n=2). One pt continues I+V (17.4 months ongoing) without further treatment. Two pts died from disease progression without receiving additional treatment.
The median OS of all 33 pts was 27.4 months (95% CI 25.9 months-NE; Figure 1B). The median OS of the 22 pts with prior progression on I (V-naïve) was 47.1 months (95% CI 27.7 months-NE), and the median OS of the 11 pts with prior progression on I and V separately was 27.0 months (95% CI 15.5 months-NE). In univariate analyses, prior progression on V was a predictor of shorter OS (HR=3.9; 95% CI 1.4-11.2; p=0.01).
CONCLUSIONS
Combination I+V therapy in heavily pre-treated CLL pts who have disease progression after I provides durable benefit with a median OS approaching 4 years. In the double-refractory setting, repurposing both drugs as combination I+V therapy can be a useful strategy, despite the short-term disease control, for pts where clinical trial options are limited or not available. Further examination of these approaches in larger cohorts and understanding the operative resistance mechanisms in pts with double-refractory disease are key next studies.
Ding: Octapharma: Membership on an entity's Board of Directors or advisory committees; DTRM: Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding. Chanan-Khan: BeiGene, Jansen, Ascentage: Honoraria; Ascentage, Starton, Cellectar, NonoDev, Alpha2 Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Alpha2 Pharmaceuticals: Patents & Royalties: Tabi; Ascentage: Research Funding; Cellectar: Current equity holder in publicly-traded company; Alpha2 Pharmaceuticals, NonoDev, Starton: Current holder of stock options in a privately-held company; BieGene, Jansen, Ascentage: Consultancy. Kenderian: Humanigen, Inc.: Consultancy, Honoraria, Research Funding. Wang: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Novartis: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees. Ailawadhi: Pharmacyclics: Consultancy, Research Funding; Xencor: Research Funding; Janssen: Consultancy, Research Funding; Cellectar: Research Funding; Sanofi: Consultancy; BMS: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Ascentage: Research Funding; Medimmune: Research Funding; Karyopharm: Consultancy; Amgen: Consultancy, Research Funding; Beigene: Consultancy; AbbVie: Consultancy; Takeda: Consultancy; Genentech: Consultancy. Koehler: AbbVie: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Kay: AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Targeted Oncology: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squib: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Research Funding; Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; CytomX Therapeutics: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Research Funding; Behring: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Tolero Pharmaceuticals: Research Funding. Parikh: Pharmacyclics, MorphoSys, Janssen, AstraZeneca, TG Therapeutics, Bristol Myers Squibb, Merck, AbbVie, and Ascentage Pharma: Research Funding; Pharmacyclics, AstraZeneca, Genentech, Gilead, GlaxoSmithKline, Verastem Oncology, and AbbVie: Membership on an entity's Board of Directors or advisory committees.
Combination ibrutinib and venetoclax in patients with relapsed CLL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal